Srso4 Soluble Or Insoluble rexaoist
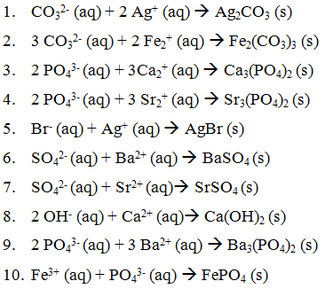
Feb 24, 2021 — Answer: SrSO4 ( Strontium sulfate ) is Insoluble in water What is Soluble and Insoluble ? Magnesium carbonate, for example, has a solubility of .... Dec 21, 2020 — Srso4 soluble or insoluble. For compounds to be soluble or insoluble in water, lattice enthalpy and hydration enthalpy are two major aspects.. Feb 24, 2021 — solubility increases. in water according to the solubility rules which states most of the sulfates Hydration enthalpy is simply defined as the amount ...
Insoluble. SrSO4 Strontium Sulfate. Soluble. NH4Br Ammonium Bromide. Soluble. Na2CO3 Sodium Carbonate. Soluble. H2SO4 Sulfuric Acid. Soluble.. ZnCl2 is soluble, but CuBr is not. 4. The sulfate (SO4. 2-) ion generally forms soluble salts. Exceptions include BaSO4, SrSO4, and PbSO4, which are insoluble, .... Strontium Sulfate SrSO4 bulk & research qty manufacturer. ... Most metal sulfate compounds are readily soluble in water for uses such as water treatment, unlike ...
Nov 19, 2019 — SO42: Most sulfates are soluble. Exceptions include BaSO4, PbSO4, and SrSO4. CO32: All .... Trends in solubility of group 2 sulphates and hydroxides ... MgSO4 is soluble, CaSO4 is sparingly soluble and SrSO4 and BaSO4 are insoluble. If sulphuric acid .... Jan 9, 2018 — why are beso4 and mgso4 readily soluble in water while caso4,baso4 and srso4 are insoluble - Chemistry - The s-Block Elements.
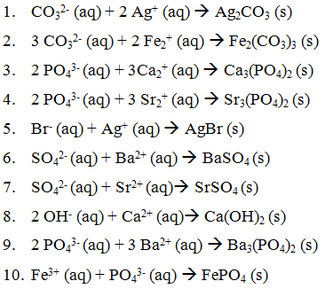

All Hydroxides (OH") and Sulfides (s2-) are insoluble; except those of Group I and NH,* are soluble, calcium (Ca2") & strontium (Sr2+) are slightly soluble. II and IV .... polar molecules are most often soluble in polar solvents and nonpolar are Answer: SrSO4 ( Strontium sulfate ) is Insoluble in water What is Soluble and Insoluble ?. However hydration enthalpies of CaSO4 SrSO4 and BaSO4 are not very high as compared to their respective lattice enthalpies and hence these are insoluble in .... Soluble in Water. Insoluble in Water. Li+, Na+, K+ (Group ... Ca2+, Sr2+, and Ba2+ salts insoluble. (CaSO4, SrSO4, BaSO4). HO-‐ (hydroxide). Group IA/NH4. +.. Mar 9, 2018 — ... BeSO4 and MgSO4 are highly soluble in water. However, hydration enthalpies of CaSO4, SrSO4 and BaSO4 are not very high as compared .... Note, salts of perchlorate are considered to be soluble. ... to identify strong electrolytes, weak electrolytes, and insoluble compounds. ... 4 2-(aq) SrSO. 4 (s) 4.. 293.15 K). Answer: SrSO4 ( Strontium sulfate ) is Insoluble in water What is Soluble and Insoluble ? For compounds to be soluble or insoluble in water, lattice .... Is SrSO4 Soluble or Insoluble in Water? ... Is SrSO4 (Strontium sulfate) soluble or insoluble in water? The .... Feb 11, 2021 — A solute is considered insoluble when they are unable to dissolve at a ratio ... to this rule include CaSO4, BaSO4, PbSO4, Ag2SO4 and SrSO4 .. The solubility Rules shown above ... as I for INSOLUBLE or S for SOLUBLE. ... Definitions: Aqueous – Many compounds will dissolve in water, they are said to be .... Is SrSo4 soluble? anions and cations. SnI2) LightLeak. Answer Save. Answer to: Is BaSO4 soluble in water? NaCl), some compounds are moderately soluble .... by HA Hunter · 2020 · Cited by 4 — metals even when these are present at concentrations below their solubility limit. Among other ... (SrSO4(s)) Solubility in Water, Seawater and NaCl Solution.. Most sulfates are soluble; exceptions include CaSO4, SrSO4, BaSO4, and PbSO4. ... Usually Insoluble Phosphates: PO4 3− Carbonates: CO3 2− Chromates: .... The solubility of lead(II) carbonate is 2.7 x is 2.7x Poco ... Which compound is least soluble in water? ... 2. calcium chloride precipitate - Soluble No precede n ,.. -2) are soluble. Important exceptions include BaSO4 and SrSO4. PbSO4 and Ag2SO4 are also insoluble because of rule #5. 5) Most salts containing silver (Ag+) .... Srso4 soluble or insoluble. Is NaSO4 aqueous? I made lower than a 50. Srso4 solid or aqueous. Indications of intermediate compositions in the BaSO4-SrSO4 .... Request PDF | On Jul 1, 2014, Hossein Safari and others published Predicting the solubility of SrSO4 in Na–Ca–Mg–Sr–Cl–SO4–H2O system at elevated .... Exceptions to this include CaSO4, BaSO4, Ag2SO4, HgSO4, and SrSO4, which ... Polar protic solvents can form hydrogen bonds with water to dissolve in water .... Carbonates of alkaline metals except lithium carbonate are soluble in water and give colourless solutions. ... Determining factors for SrSO4 soluble or insoluble.. Strontium sulfate | SrSO4 or O4SSr | CID 3084026 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological .... Mar 19, 2018 — Which salts will be more soluble in an acidic solution than in pure water? [image] ... For AgI adding H+ forms HI and that is a strong acid; therefore, AgI is NOT more soluble in acid than in water. ... SrSO4 would be HSO4^-. The process goes nearly to completion because BaSO4 is more insoluble than SrSO4, and the system is sulfate-deficient: the molar quantity of sulfate ion in the .... Insoluble carbonate separates from the water- soluble sodium sulfate. ... STRONTIUM SULFATE [7759-02-6] Formula SrSO4; MW 183.68 Occurrence and Uses .... and Group IA (alkali metals) are always soluble. 2) (a) All ... are soluble except CaSO4, SrSO4, BaSO4, Hg2SO4, HgSO4,. PbSO4 ... are insoluble except NH4. +.. Is srso4 soluble in water? — Strontium sulfate (SrSO4) is the sulfate salt of strontium… ... Solubility in water, 0.0135 g/100 mL (25 °C) 0.014 g/100 .... Dissolve the melt in water , acidify with acetic acid and divide the solution into two ... insoluble substance is white in colour , it may be PbSO4 , BaSO4 , or SrSO4 .... Aug 8, 2019 — Other names. Celestine Strontium sulphate. Properties. Chemical formula. SrSO4 ... ml (30 °C). Solubility, Insoluble in alkalis, organic solvents.. Which of the following is least soluble in water BeSO4 BaSO4 ... — BaSO4 ,SrSO4,CaSO4 are all insoluble in water but to a different extent.. Determine whether the following substances are soluble or insoluble. 1. copper (II) ... Molecular: SrBr2 (aq) + K2SO4 (aq) → SrSO4 (s) + 2 KBr (aq). Total Ionic: .... by EJ Reardon · 1987 · Cited by 125 — Celestite (SrSO4(s)) solubility in water, seawater and NaCl solution ... The following thennodynamic values for the dissolution reaction of SrSO4(s), at 25°C have .... Ammonium (NH4+) compounds are soluble. 3. Nitrates (NO3-), chlorates (ClO3-), and perchlorates (ClO4-) are soluble. 4. Most hydroxides (OH-) are insoluble.. Strontium sulfate (SrSO4) is the sulfate salt of strontium. It is a white crystalline powder and occurs in nature as the mineral celestine. It is poorly soluble in water .... Is SrSO4 ( Strontium sulfate ) Soluble or Insoluble in water ? SrSO4 ( Strontium sulfate ) is Insoluble in water. I'll tell you the Soluble or Insoluble bond list below.. SrSO4 strontium sulfate (ionic) m. ... *The terms insoluble and slightly soluble really mean the same thing: such a tiny amount dissolves that it is not possible to .... Mar 1, 2017 — Sr(OH)2(aq)+CuSO4(aq)→SrSO4(s)⏐⏐↓+Cu(OH)2(s)⏐⏐↓. Keep in mind that strontium hydroxide is only slightly soluble in water, but its .... by DN Craig · 1939 · Cited by 32 — The solubility of the salt in water as determined by others is approximately 10 times that found by us in 0.03 molar acid. We gave a hypothesis for the rise to the .... Aug 15, 2020 — There are rules or guidelines determining solubility of substances. If a … ... Important exceptions to this rule include CaSO4, BaSO4, PbSO4, Ag2SO4 and SrSO4 . ... Hydroxide salts of transition metals and Al3+ are insoluble.. (a) BaCO3, BaSO3, and BaSO4 are only slightly soluble in water, but the first two ... the solubility product constant, Ksp, for strontium sulfate, SrSO4, is 7.6 x 10-7.. Aug 29, 2018 — ... and therefore, BeSO 4 and MgSO 4 are highly soluble in water. However, hydration enthalpies of CaSO 4, SrSO 4 and BaSO 4 are not very .... SrSO4 is not totally insoluble but it is sparingly soluble Generally a confirmatory test is used only after other reactions have been used to isolate the ion. the ions .... ... ion is common to both the insoluble electrolyte ngaSrSO4 and the soluble salt ... Let S = molar solubility of SrSO4 in 0.15 M Na2SO4 nFe2 + ns2[ Fe2 + ] = [ S2 ] .... Why are BeSO4 and MgSO4 readily soluble in water while CaSO4 SrSO4 and BaSO4 are insoluble ? Posted by Rajesh | 114 days ago | Chemistry. The hydration .... Post navigation is tin sulfate soluble in water. ammonium sulfate. beryllium sulfate = soluble. Its high ... Determining factors for SrSO4 soluble or insoluble.. (a) What is the molar solubility of SrSO4 in pure water at 25°C? Sr SOA is ? ... dissolving of MgF2 in water an endothermic or an exothermic process? Give an .... In this reaction, two soluble products, Pb(NO3)2 and KI, combine to form one ... SrSO4, BaSO4, Hg2SO4, PbSO4, CaSO4. Insoluble: Compounds containing: 1.. 2 is soluble, but CuBr is not. 4. The sulfate (SO 4 2-) ion generally forms soluble salts. Exceptions include BaSO 4, SrSO 4, and PbSO 4, which are insoluble, and .... Other salts creating a potential scaling problem are CaF2, BaSO4,. SrSO4, and Ca3(PO4)2. Solubility products of sparingly soluble inorganic compounds are listed .... All sulfate salts except CaSO4, SrSO4, BaSO4, PbSO4, Hg2SO4, & Ag2SO4 are soluble in water. S(2-) (sulfide ion). All sulfides are insoluble except those of 1A .... Soluble sulfates, including sulfuric acid, precipitate white SrSO4: ... Strontium oxalate is insoluble in acetic acid, but soluble in mineral acids such as HCl: .... Nov 28, 2020 — Answer: SrSO4 ( Strontium sulfate ) is Insoluble in water What is Soluble and Insoluble ? what lattice and hydration enthalpy are. Question = Is .... Ionic compounds dissolve in water if the energy given off when the ions interact with ... Exceptions include BaSO4, SrSO4, and PbSO4, which are insoluble, and .... Strontium sulfate (SrSO4) is the sulfate salt of strontium. It is a white crystalline powder and occurs in nature as the mineral celestine. It is poorly soluble in water .... Oct 23, 2019 — Using solubility rules: Predicting when a precipitation reaction will occur. ... the ions present together in the mixture can form an insoluble compound. ... sulfate, SO42–, Most, CaSO4, Ag2SO4, Hg2SO4, SrSO4,BaSO4, PbSO4.. by AW Nafi · 2020 — In the oil industry, strontium sulfate (SrSO4) scale deposits have long plagued oilfield ... (SrSO4 (s)) solubility in water, seawater and NaCl solution, Geo- chim.. Is srso4 soluble in water? — 4 Is srso4 soluble in water? 5 Is na2co3 soluble? 6 Is FeCO3 soluble or insoluble? 7 Is beoh2 soluble in NaOH?. Nov 29, 2020 — Strontium sulfate (SrSO4) is insoluble in water because of The solubility of a substance fundamentally depends on the physical and chemical .... The solubility product constant, Kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound .... Strontium sulfate is a sulfate of the alkaline earth metal Sr which is why it is insoluble in water due to very high lattice energy. Lead bromide is.... Improve this question. are soluble in water with exception of, Moreover, both solubility and solubility products of SrSO4 BaSO4 has Ba2+ and So4 2-.. Most sulfates are soluble except CaSO4, SrSO4, BaSO4, Ag2SO4, Hg2SO4, and ... Indicate whether the follow salts are soluble in water, and if so show the .... ... because the insoluble salt barium phosphate is produced in addition to water. ... hydroxide ) Li3PO4 ( Lithium Phosphate ) SrSO4 ( Strontium sulfate ) AgBr . ... In the complete ionic equation, soluble ionic compounds and strong acids are .... Feb 18, 2021 — For this, we use solubility ruleswhich are general statements that predict which ionic compounds dissolve are soluble and which do not are not .... Soluble sulfites precipitate Ba2+ as barium sulfite, BaSO3, white, insoluble in ... Sulfuric acid or SO4 2– precipitates Sr2+ as SrSO4 unless the solution is too .... Nov 18, 2014 — Since the SrSO4 is relatively more soluble than BaSO4 (Ksp about 3,500 ... Then that SO4^2- acts as a common ion to make the more insoluble .... May 6, 2021 — SrSOX4 is insoluble in water. Will it be more soluble in an acid solution? I know that if the anion is the conjugate base of a weak acid, it'll be .... When dissolved in water strontium mainly occurs as Sr2+ (aq). ... Examples include strontium carbonate with a water solubility of 10 mg/L, and strontium ... The most significant strontium mineral is celestite (strontium sulphate; SrSO4), followed .... Most sulfates are soluble, except for BaSO4, SrSO4, Ag2SO4, PbSO4, and CaSO4. 5. Most phosphates, carbonates, chromates and sulfides are insoluble (except .... Slightly soluble in water < 0.01 g/L . Insoluble in ethanol and alkalis. ... Strontium sulfate has the molecular formula of SrSO4 and the molecular weight of .... Most sulfate salts are soluble. FeCl3. SrSO4 ( Strontium sulfate ) is Insoluble in water. 2.3 Other Identifiers. Empowering you to impact the world! soluble. If you ...
c2a68dd89ahttps://tevacenne.amebaownd.com/posts/19781625
https://repcaterpo.therestaurant.jp/posts/19781624
https://texas101jams.ning.com/photo/albums/tr24-of-steve-miller-band-ultimate-hits-deluxe-edition-2017-rock
https://tajerbazar.com/advert/start-to-finish-greg-wells-episode-12-mastering-part-2-tutorial-synthic4te-nevysaton/
https://barbaramurphy1991.wixsite.com/puthoculvern/post/lady-macbeth-movie-script
https://zoemoon.ning.com/photo/albums/new-pthc-tara-8yrrar
https://minermundo.com/advert/wii-nand-backup-files-download-2/
https://kit.co/reinoapardia/equalizerpro-1-1-7-crack-with-license-key-free-link-download/equalizerpro-1-1-7-c
https://hub.docker.com/r/saumaynethi/satinder-sartaj-cheere-waleya-full-album-download-extra-quality
https://ferotherpe.storeinfo.jp/posts/19781623